|
|
Leiomyoma at the Ampulla of Vater:
A Case Report |
*Dr. Rayadh A. Zaydan. FIBMS.GIT.
**Dr.Luay E . Al-Khurry. Msc. Path.
***Dr.Khitam R. Al-Khafaji. FIBMS. Path.
****Dr.Suhair A. Al-Salihi. FIBMS. Path.
*Medical Dep. Gastroenterology hepatology teaching hospital. Medical city.
**Pathology Dep. College of Medicine, Baghdad University, Iraq.
***Lab Dep. Gastroenterology and hepatology teaching hospital. Medical city.
|
 |
| ABSTRACT |
 |
| We describe a case in which transduodenal ampullectomy was performed for leiomyoma. A 19 years old female was found to have a tumor at the distal end of the common bile duct (CBD). Excision of the ampulla showed a well defined mass 2x1cm size and histopathological study revealed a benign smooth muscle tumor, leiomyoma. |
 |
| INTRODUCTION |
 |
| Gastrointestinal stromal tumors (GISTs) are a large family constituting the majority of non epithelial neoplasm of gastrointestinal tract (GIT). They may also involve the omentum, mesentry, and retroperitoneum(1). Gastrointestinal stromal tumor rarely develops in the duodenal ampullary region (2). After a thorough and comprehensive literatures review, we have found that this is the first time in which benign leiomyoma is described in the ampulla of Vater since the previous reported cases were malignant GISTs (2,3, 4) . |
 |
| CASE REPORT |
 |
A nineteen years old female was admitted to our hospital complaining of jaundice lasting more than six weeks associated with low grade fever. Ultrasound showed enlarged liver, normal texture, with no space occupying lesion, dilated intra and extra hepatic biliary duct, CBD was dilated till distal end. OGD endoscopy was performed and there was pangastropathy with prominent ampulla. Then Endoscopic ultrasound (EUS) showed a hypoechoic homogenous mass like lesion 2x1.3cm in the ampullary area without evidence of invasion of duodenal wall or pancreas, the mass was completely occluding the CBD but not the pancreatic duct (Fig1). Transduodenal ampullectomy was performed and the Histopathological study showed well circumscribed tumor nodule composed of uniform spindle cells arranged in whorls and fascicles with eosinophilic cytoplasm and cigar shaped elongated nuclei. The mitotic figure was negligible and there was no tumor necrosis. (Fig2).
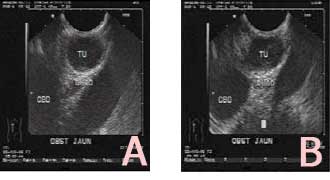
Fig 1: A, a hypoechoic mass at the ampullary region surrounded by duodenal wall layers without evidence of invasion of duodenal wall or pancreas. B, the tumor is completely obstructing the distal end of the common bile duct.

Fig 2: A, a well circumscribed tumor nodule lying below the ampullary surface epithelium. B, the tumor is composed of spindle cell proliferation arranged in whorls and fascicles. Inset, a high power view showing uniform cells with elongated nuclei. |
 |
| DISCUSSION |
 |
| GIST may arise anywhere in the alimentary tract and frequently are incidental findings on radiologic and endoscopic examination. Endoscopic ultrasound (EUS) is the investigative procedure of choice when a submucosal lesion has been visualized endoscopically (5). EUS can accurately show the exact origin of a lesion, whether inside or outside the GIT wall. Inside the GI wall EUS can detect the layer of origin, for instance the 4th layer in leiomyoma or the 3rd layer in lipoma. High frequency ultrasound probe enables proper diagnosis of esophageal leiomyoma derived from muscularis propria (6). Unfortunately, EUS can not reliably show the difference between the benign and malignant submucosal tumors (7). However characteristics such as size, border, homogeneity and presence of necrosis can help to decide whether a lesion should be surgically removed or to be followed up by EUS. Nevertheless, the diagnosis on the basis of EUS is perceptive and can not replaces the histopathological diagnosis of GIST (8).In the past most of them were diagnosed as leiomyoma or leiomyosarcoma (9). Recently they have been classified as c-kit or CD34 positive mesenchymal tumors based on immunohistochemical and electron microscopic approaches (10). Most GISTs are diagnosed histopathologically after resection because of submucosal location (3). They may grow expansively without being invasive and sometimes metastasize to the liver and recur locally (11, 12). Surgery with safe surgical margins and no tumor rupture is necessary and adequate means of treating such tumors. Excessive lymph node dissection is unnecessary, because GIST rarely metastasize to regional lymph nodes (13). These tumors can be roughly divided into four major categories on the basis of their phenotypic features: 1) tumors showing differentiation toward smooth muscle cells; 2) tumors with apparent differentiation toward neural element; 3) tumors showing dual differentiation toward smooth muscle and neural elements; 4) tumors lacking differentiation toward either cell type (9, 10). Malignant risk categories for GISTs are based on tumor size and mitotic activity, and are divided into very low (benign), low (of undetermined malignant potential), intermediate (low grade malignant), and high risk (high grade malignant) categories (14, 15). In our case the size of the tumor was taken into consideration and depending on the histopathological features the resultant diagnosis was ampullary GIST of a very low risk of malignancy (leiomyoma). So despite of its rarity this neoplasm should be included in the differential diagnosis of the tumors appearing in the duodenal ampullary region. |
 |
| REFERENCES |
 |
- Reith JD, Goldblum JR, Lyles RH, Weiss SW. extragastrointestinal (soft tissue) stromal tumor: an analysis of 48 cases with emphasis on histotlogic predictors of outcome. Mod Pathol 2000, 13: 577-585.
- Matsushita M, Kobayashi Y, Kobayashi H, Nagasawa M, Sato Y, Nakamura H. A case of gastrointestinal stromal tumour of the ampulla of Vater. Dig Liver Dis. 2005 Apr; 37(4):275-7. Epub 2005 Jan 8.
- Kim SH, Kim JH, Baik GH, Baek I, Hahn T, Oh SO, Kim JB, Park SH, Chang WK, Kim DJ, Park CK, Park HR. Malignant gastrointestinal stromal tumor of the ampulla of Vater: a case report. Korean J Gastroenterol 2004 Jan; 43(1):66-70.
- Takahashi Y, Noguchi T, Takeno S, Uchida Y, Shimoda H, Yokoyama S. Gastrointestinal stromal tumor of the duodenal ampulla: report of a case. Surg Today. 2001; 31(8):722-6.
- M. Matusi, H. Goto, Y. Niwa, et al. preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumor. Endoscopy 1998; 30: 750-755.
- Takadad N, Hrgashino M, Osugi H, et al. Utility of endoscopic ultrasonography in assessing the indication for endoscopic surgery of submucosal esophageal tumor. Surgical endoscopy 1991; B 282-290.
- Yamada Y, Kide M, Sakuchi T, et al. A study on endoscopic ultrasonography. Dig endoscopy 1992; 4: 396-408.
- Lee S.J, Park S.W, Song J.B. the diagnostic value of endoprobe by continuing water infusion method for mucosa. Gastrointestinal endoscopy 2002; 56: S102.
- Pidhorechly I, Cheney RT, Lraybill WG, Gibbs JF. Gastrointestinal stromal tumors: Current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000; 7:705-12.
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983; 7:507-19.
- Pieri JPEN, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 2001; 136:383-9.
- Dematteo RP, Lewis JJ, Leung D, Mudan S, Woodruff J, Brennan MF. Two hundred gastrointestinal stromal tumors. Recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231:51-8.
- Rosai J. Rosai and Ackerman,s Surgical Pathology; 9th ed,Mosby.2004.
- Chandrasoma P.Gastrointestinal pathology; Appleton & Lange.1999.
- Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, rubin BP, Shmookler B, Sobin LH, Weiss SW. diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Patho; 2000, 33: 459-465; Int J Surg Pathol 2002, 10: 81-90.
|
|
|


